Learn more about the academic programs we are delivering in Summer 2024. If you have any questions about part-time studies, please contact us.
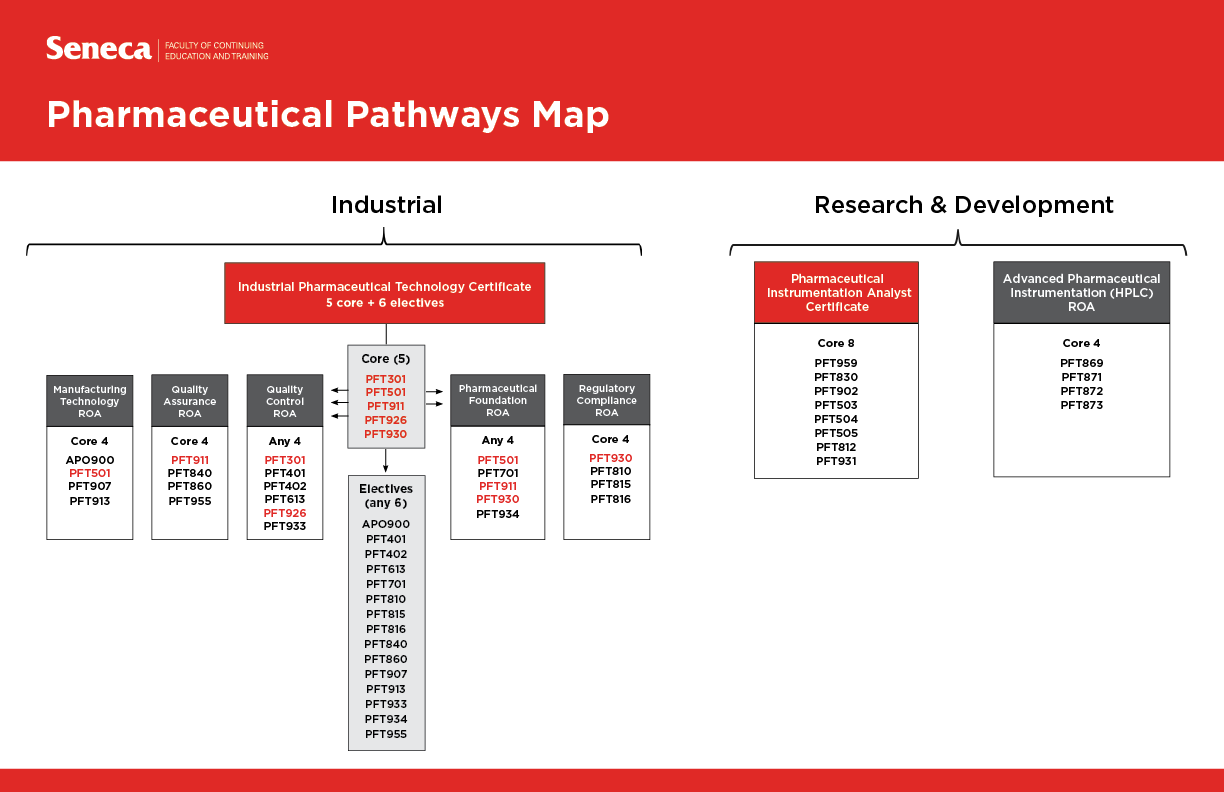
Industrial Pharmaceutical Technology - For Apotex Employees only
Seneca College Certificate
Overview
Please note: this program is restricted to Apotex employees only. Anyone registered who is not associated with Apotex will be withdrawn.
This certificate program is designed to meet the training needs of Apotex employees working in various pharmaceutical departments who need to upgrade their existing skills or acquire new skills and knowledge in specific areas of the industry. This comprehensive certificate program provides training in pharmaceutical analysis, product formulation, quality assurance and manufacturing, pharmacology and toxicology.
Students must wear lab coats and safety glasses in the lab.
Entry Requirements
Ontario Secondary School Diploma University/College Preparation: Grade 12 Chemistry, Mathematics, English and Grade 11 Biology are required. Mature students with work experience are considered on that basis, but may be advised to take College Preparatory Chemistry, Biology, Math and English (refer to College/University Prep section).
Part-time Studies courses are being offered in either of the following four formats: Online, Flexible, In-person, Hybrid. Click Availability below to see current offerings.
Curriculum
Please note: this program is restricted to Apotex employees only. Anyone registered who is not associated with Apotex will be withdrawn.
This course is an introduction to the methods related to the production of pharmaceuticals. Several dosage forms such as solids, liquids, semi-solids, and parenterals are discussed. In each, the merits of the type, composition, handling, processing, testing and equipment used are discussed to provide a foundation from which more specific training can be undertaken. Regulatory requirements which serve as controls, as well as the rationale which support their application are discussed and applied to areas of plant operations. (Lecture only)
This subject introduces students to the fundamental pharmacological aspects of drugs and their interaction with various types of cells. Topics include routes of drug administration, factors affecting drug absorption and mechanism of action. Specific drugs for the central and autonomous nervous systems as well as the cardiovascular system are studied. Drugs for the endocrine system, therapeutic monitoring system and antiallergics, antihistaminics are also covered. (Lectures only)
PFT930 - Industrial Drug Legislation
This course describes the role of the Regulatory Affairs department in a pharmaceutical firm and focuses on the "submission process" - the regulatory requirements at the various stages in a drug product's development from inception through IND (Investigational New Drug) and NDS (Notice Of Compliance). The functions of relevant divisions of the Health Protection Branch are discussed. Also covered are: DINs (Drug Identification Numbers), Provincial Formulatory Submissions, drug labelling and drug advertising, SOPs (Standard Operating Procedures), compliant handling (including Adverse Drug Reactions), recalls, Plant Master Files (PMF), Drug Master Files (DMF) and regulatory inspections. (Lecture only)
PFT/APT810
This subject focuses on product development and registration in Canada. Students learn, and get a hands on approach to prepare a high quality clinical trial application (CTA), including generic and brand submissions. Emphasis is on the mechanics of preparing submissions in the Common Technical Document (CTD) format in Canada. Specifically, this subject clarifies the rules, regulations and guidelines on how submissions are prepared, and how deficiency letters are addressed to TPD. An overview of the e-CTD modules is provided in detail. (Lectures Only)
PFT/APT815 - Advanced Regulatory Affairs
This course provides students with a review of the functions and purposes of international harmonization and International Regulatory Affairs. Pharmaceutical regulations in USA, European countries, Japan and MENA region are examined as well as the regulations in a selected number of other geographic locations. Drug submission requirements based regulations in these countries are compared.
This course describes the role and responsibilities of the Quality Assurance department in a pharmaceutical company. A) It focuses on the design of proper quality control/quality assurance systems to ensure the manufacturing of safe products and also to satisfy regulatory authorities. All systems, from cleaning to component testing, from product assay to product release are geared to provide the manufacturer documented quality checkpoints on the product. B) It focuses on the management of the quality assurance systems to provide sufficient and adequate records on all testing distribution and all other procedures that must be in place. (Lecture only)
This course is an overview of the various aspects of validations, qualification/validation of pharmaceutical systems, processes and equipment. These processes received acceptance by the pharmaceutical industry in the late 1970s to ensure current Good Manufacturing Practices (cGMP) and product quality and integrity. Reliance upon end-product testing is insufficient to assure product quality. A high degree of quality of assurance arises only from good product and process design, validation and process controls. Validation concepts are applicable to both oral, solid dosage forms and sterile manufacturing pharmaceutical facilities. (Lecture only)
PFT/APT501
This subject is designed as a continuation of PFT501- Pharmaceutical Manufacturing Methods, teaching advanced pharmaceutical dosage forms in respect to design, development, techniques and technology involved. The processes and methodology involved in manufacturing of different dosage forms are compared in the following perspectives: equipment, benefits and appropriate application. (Lecture only)
This subject focuses on the concept of quality assurance and the contributions of quality assurance in a pharmaceutical organization. It takes a snapshot examination of common quality applications, tools, processes, and systems relevant to the pharmaceutical industry. Topics covered include: foundations of quality, basic statistical concepts and applications, document systems, SOPs, quality audit, validation, ISO standards, GMPs (FDA and HPFBI), product recall and complaints handling.
This course deals with experimental investigation and evaluation of many kinds of package containers such as glass, plastic, metal and paper, closures, liners, and rubber stoppers. Topics include testing methods, development of quality control methodology, legislative influences on packaging technology, and the role of the package in marketing. (Lecture only)
It is strongly recommended that students have basic computer skills as this course requires computer access.
This course examines the organizational role, responsibilities and requirements of the Health Canada related to the production, labelling, selling, and advertising of drugs, radiopharmaceuticals, biologics, natural health products and medical devices in Canada. A review of USA and EU laws and regulations is also covered. (Lecture only)
This course covers the principles of contamination control in pharmaceutical manufacturing. Topics include: basic microbiology, government regulations related to contamination control, microbial control of contamination in manufacturing, air filtration, ventilation, purification and control of water system and water testing for pharmaceutical manufacturing, production hygiene, employee health and hygiene requirements, microbiological testing of environment and interpretation of critical test results, evaluation of disinfectant and cleaning validation, self inspection and training of production employees for contamination control. (Lecture only)
Program Outcomes
Upon successful completion of this program, students are able to:
- Perform an analysis of pharmaceutical substances in order to analyse quality, purity and strength of a given product
- Describe the formulation and process aspects of pharmaceutical products
- Perform drug assays
- Validate pharmaceutical systems, processes and equipment
- Apply knowledge of packaging technology and quality control to pharmaceutical production
- Apply principles of microbiology and pharmaceutical chemistry to ensure safety and sanitation related to pharmaceutical manufacturing
- Apply knowledge of clinical pharmacology to the development of drugs
- Describe the submission requirements related to a new drug, the approval process, drug registration and ongoing regulatory requirements (complaint handling, inspections recalls, for example)
- Comply with relevant Good Manufacturing Practices (GMP), local as well as international regulatory requirements and drug legislation
- Design a quality assurance (QA) system and apply quality assurance/control measures to pharmaceutical manufacturing and packaging
- Apply knowledge of dosage form to manufacturing and quality control of pharmaceutical products
- Perform as a member of a quality assurance team
- Properly use and maintain both a liquid and gas chromatographer
- Control contamination during the manufacture of pharmaceutical products
Credit for Prior Learning
Prior Learning Assessment
Earn college credits for what you already know.
Prior Learning Assessment is a method of assessing and recognizing learning that is equal to
college level learning, but has been gained outside a traditional classroom (through work
experience, volunteering, outside study, etc.). If you can prove that the knowledge you have gained
meets the outcomes of a Seneca course, then credit will be awarded.
How does the PLA process work?
Prior Learning is demonstrated through a "challenge" process. The process measures learning
through a variety of methods which may include tests, portfolio assessment, interviews,
demonstrations, essays, and work samples. The method used will be determined in consultation with a
Program Coordinator.
For more information and to determine if you are eligible for PLA, please call the Program
Coordinator.
The process may take from 6 to 8 weeks.
Note: Not all courses can be challenged. For more information go to PLA website or contact your Program Coordinator.
Transfer Credit
Many students who enter Seneca Polytechnic will have earned academic credits in post-secondary educational institutions which they may be able to apply toward completion of a Seneca College program.
Requests for Transfer Credit must be for a specific course and must be accompanied by an official transcript and course outline. A minimum grade of "C" (60 percent) is generally required for a course to be considered for Transfer Credit.
Download a Transfer Credit Request form. An official copy of your transcript and applicable detailed course outlines should be attached and submitted. Please note it may take 4 to 6 weeks for a Transfer Credit decision.
More Information
Please visit the Office of the Registrar.
Graduation/Convocation
When you meet all program requirements and become eligible for a certificate, diploma, or degree, you must inform the Registrar by completing a Graduation Application form and paying the graduation and alumni fee. Certificates, diplomas, and applied degrees are issued twice a year in the Fall (October), Spring (June) and Winter (February).
For further information including deadlines and fees, please visit the Convocation website or contact the Convocation Office at theservicehub@senecapolytechnic.ca.
Minimum Performance for Graduation
A student will be eligible to graduate from a certificate, diploma, advanced diploma or graduate certificate program if they have achieved a minimum graduating GPA of 2.0.
A student will be eligible to graduate from a degree program if they have achieved a minimum graduating GPA of 2.5, which includes a minimum GPA of 2.5 in the courses in their main field of study and a minimum GPA of 2.0 in breadth courses.
Residency Requirements
A faculty of Seneca College may recommended a student for a certificate, diploma or degree only after the student has earned a minimum of twenty-five percent of the credit for that program at Seneca.
Program Contacts
Maria Graziosi
Program Assistant
Maria.Graziosi@senecapolytechnic.ca
416-764-0964
Melanie Rubens
Program Coordinator
Melanie.Rubens@senecapolytechnic.ca
416-764-0973
For more information about this program, fill out the following form.